ALACHUA, Fla., Nov. 21, 2016 (GLOBE NEWSWIRE) — AxoGen, Inc. (NASDAQ:AXGN), a global leader in innovative surgical solutions for peripheral nerve injuries, today announced the commercial release and first clinical implant of Avive™ Soft Tissue Membrane. Avive™ Soft Tissue Membrane is a minimally processed human umbilical cord membrane delivering the benefits of amnion to reduce inflammation and scar tissue formation.
Avive™ represents AxoGen’s fourth surgical product and may be used in conjunction with nerve surgery techniques to proactively reduce inflammation and scarring. With the addition of the market opportunity for Avive™, the Company now estimates that the current addressable market of AxoGen’s nerve product portfolio has increased from $1.6 billion to $1.8 billion. Today the Company also announced that its salesforce has increased to 51 representatives, up from 45 at the beginning of November, supporting the continued penetration of AxoGen’s solutions in the peripheral nerve repair market.
“We are pleased to further expand our portfolio of products with the release of Avive™ Soft Tissue Membrane,” commented Karen Zaderej, AxoGen’s President and Chief Executive Officer. “Providing surgeons with a proactive solution to some of the common challenges they experience strengthens our position as the preeminent company in peripheral nerve surgery.”
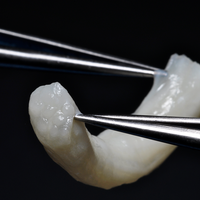
Post-operative scar tissue and adhesion development is an inherent complication to nerve tissue following traumatic injury or surgical intervention. Used on injured nerves or surrounding soft tissues, Avive™ is a resorbable covering to keep potentially adherent surfaces apart. Processed from umbilical cord, Avive™ Soft Tissue Membrane is up to eight times thicker than amniotic sac membrane allowing for ideal handling, suturability and performance. Avive™ is minimally processed to ensure preservation of the natural properties of umbilical cord amniotic membrane. It stays in place during the critical phases of tissue regeneration to protect the tissue from inflammation and scarring.
The first use of the Avive™ Soft Tissue Membrane was performed at The Buncke Clinic in San Francisco by Dr. Bauback Safa, M.D. F.A.C.S. and Dr. Andrew Watt, M.D. The implant was used in a revision of a crushed forearm.
“Avive™ provides a novel solution to a challenging problem,” commented Dr. Safa. “Following traumatic injury, we are often concerned with potential scarring, adhesions and tethering of the nerve. Avive™ offers a material with the ability to reduce inflammation and scarring with handling properties that are ideal for applications in nerve surgery.”
The Company will be commenting on the launch of Avive™ Soft Tissue Membrane as part of the previously announced Analyst and Investor Event being held today in New York City. The program portion of the event will be webcast live beginning at 12:15 pm and can be accessed through the Investors page at www.axogeninc.com. For more information or to RSVP please contact AxoGenEvents@troutgroup.com. For those not available to listen to the live broadcast, a replay will be archived for 90 days and available through the Investors page on www.axogeninc.com.
About AxoGen Inc.
AxoGen Inc. (NASDAQ:AXGN) is a global leader in innovative surgical solutions for peripheral nerve injuries. AxoGen’s portfolio of products includes Avance® Nerve Graft, an off-the-shelf processed human nerve allograft for bridging severed nerves without the comorbidities associated with a second surgical site, AxoGuard® Nerve Connector, a porcine submucosa extracellular matrix (“ECM”) coaptation aid for tensionless repair of severed nerves, AxoGuard® Nerve Protector, a porcine submucosa ECM product used to wrap and protect injured peripheral nerves and reinforce the nerve reconstruction while preventing soft tissue attachments and Avive™ Soft Tissue Membrane, a minimally processed human umbilical cord membrane that may be used as a soft tissue covering to reduce inflammation and scar tissue formation. Along with these core surgical products, AxoGen also offers AxoTouch™ Two-Point Discriminator and AcroVal™ Neurosensory & Motor Testing System. These evaluation and measurement tools assist healthcare professionals in detecting changes in sensation, assessing return of sensory, grip and pinch function, evaluating effective treatment interventions, and providing feedback to patients on nerve function. The AxoGen portfolio of products is available in the United States, Canada, the United Kingdom and several European and international countries.