
Sharma Laboratory
Biomaterials and Drug Delivery Systems
Targeted Drug Delivery
Despite advances in drug discovery and cell-based products, effective therapies for osteoarthritis (OA) remain elusive. OA is highly complex degenerative joint disease with maladaptive processes occurring within different tissues in the joint (eg. cartilage, synovium, and bone), each with their own unique drug delivery barriers and considerations. The Sharma Laboratory seeks to overcome barriers to effective drug delivery within the joint, by exploiting specific nanoparticle-extracellular matrix interactions and nanoparticle-cell interactions to improve tissue targeting and retention of therapeutic molecules within OA joints.
Current Projects
Magnetic particle imaging for nanoparticle tracking
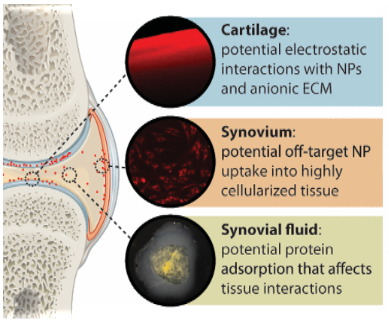
Numerous drugs are currently under investigation as osteoarthritis (OA) therapeutics, but there is a lack of effective delivery systems to improve therapeutic retention and efficacy. Nanoparticles are a promising tool to improve intra-articular drug delivery. It is important to characterize how nanoparticle behave after intra-articular delivery to the joint. Current techniques to quantify and track nanoparticles in vivo are limited. This results in inadequate understanding of how nanoparticles behave, specifically in terms of its retention, clearance, and localization to the joint. To address this limitation, this project focus on engineering magnetic nanoparticles with targeting properties to joint cartilage. Its magnetic properties allow for utilization of an emerging imaging modality- Magnetic Particle Imaging (MPI) to effectively track and assess how NPs behave in the joint. With the application of MPI, we can effectively understand particle fate over time, which is important for attaining insights about drug profiles, and developing strategies to improve therapeutic impact.
Figure: Nanoparticle (red) interactions with joint cells and tissues.

Animal models to advance understanding of PTOA progression
The use of animal models serves as critical pre-clinical tool to study the relationship between disease pathogenesis and related OA symptoms. Common animal model of OA relies on surgical methods to initiate OA. However, these models lack clinical translation and fail to capture the mechanical trauma to the joint, that induces post traumatic osteoarthritis (PTOA). Being said, this project focus on the development and characterization of a non-invasive knee injury model (NIKI) of PTOA. This is achieved by characterization of animal behavior via gait analysis (in collaboration with Dr. Kyle Allen), inflammatory makers, and evaluation joint modeling changes via histology and bone morphology changes.
Figure: Characterize inflammation after joint injury.
Scavenging for reactive oxygen species in OA
Joint injuries often lead to increased presence of reactive oxidative species (ROS) within the joint due to chronic increases in mechanical stress on cells. When intrinsic antioxidant mechanisms are not able to compensate for increased presence of ROS, oxidative stress occurs. The products of oxidative stress, including oxidation of proteins, degradation products,cell death and release of cellular contents, leads to stimulation of inflammatory cytokines by cells in the joint.
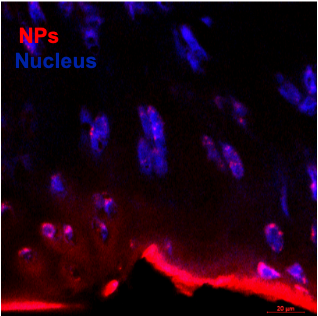
Bioactive nanoparticles made of manganese dioxide (MnO2) are being formulated in the Sharma Lab to penetrate into cartilage and demonstrate prolonged joint retention. These nanoparticles act as both drug delivery system and therapy as a treatment for post traumatic osteoarthritis. The nanoparticles are being tested with an in vitro cytokine-challenged cartilage model and an in vivo disease model and evaluated for providing protection from inflammation-induced oxidative stress.
Figure: Antioxidant nanoparticles (red) target cartilage.