
Sharma Laboratory
Biomaterials and Drug Delivery Systems
Cell-Based Therapies
A hallmark of cancer is escape from immune surveillance. Therapies aimed at reactivating the immune system have seen promising results in certain types of cancer, but there remains high variability in response rates and serious safety issues. Thus, there is an emerging interest in developing therapies that enhance the ability of the body’s own natural killer (NK) cells, which are immune cells that have an inherent ability to recognize cancer cells and destroy them without the safety issues associated with current therapies. A critical hurdle associated with NK cell therapies for solid tumors is poor tumor infiltration and cytotoxic function in the tumor microenvironment. The Sharma laboratory seeks to understand how the tumor microenvironment impacts NK cell migration and activation, and develop strategies to overcome NK cell immunosuppression to destroy tumors.
Current Projects
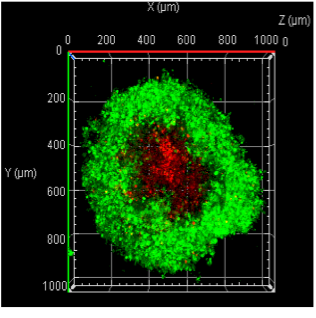
Investigating natural killer cell function in engineered 3D tumor models
NK cells are among the first-responder immune cells that recognize abnormal cells and destroy them. However, solid tumors establish a complex ecosystem, known as the tumor microenvironment, that suppress NK cell functions. The tumor microenvironment can alter both cancer cells and immune cell behavior; however, further investigation is needed to understand whether aspects of the tumor microenvironment act as a friend or a foe. We seek to understand the mechanisms by which the tumor microenvironment impacts the homing and anti-tumor activity of NK cells.
Figure: Engineered NK cells (green) attack tumor spheroid (red).

Probing mechanisms driving natural killer cell migration
NK cells have emerged as powerful tool for immunotherapy, as they rapidly recognize and lyse cancer cells without prior antigen priming. However, a critical hurdle with NK cell therapies is inadequate infiltration and activation in the solid tumor microenvironment. Yet, factors regulating NK cell tumor infiltration remain largely unknown. The long-term goal of this research is to develop biomimetic models of the solid tumor microenvironment that allow the quantification of NK cell migration and uncover mechanisms of NK cell infiltration into solid tumors.
Figure: NK cells (green) migrating through tumor models.
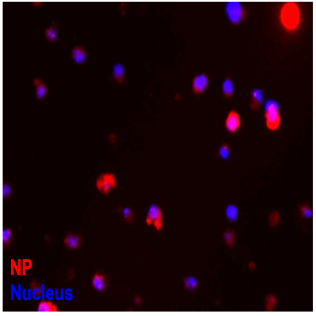
Using nanoparticle targeted delivery to activate natural killer cells
With the growing interest in utilizing biomaterials to improve cancer immunotherapies, this project is focused on exploring how nanoparticles are taken up by immune cells. NK cells can innately target and kill tumor cells, but face barriers in solid tumors that nanoparticles are able to help them overcome. Looking at how these nanoparticles are taken up can help us develop new nanoparticle systems to improve cell-based immunotherapy outcomes.
Figure: Size dependent uptake of nanoparticles (red) by NK cells
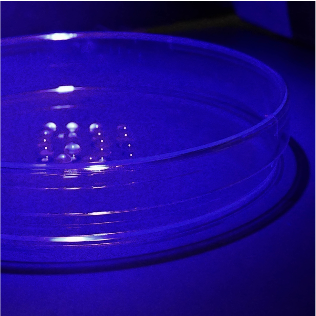
Recapitulating aspects of the tumor microenvironment
Currently 2D preclinical cancer models lack interactions that are crucial for tumor development and progression. The need for 3D cancer models is evident in order to have more biologically relevant systems for drug development. We are tissue engineering 3D models of the tumor microenvironment to interrogate how specific biochemical and mechanical cues impact NK cell migration and interaction with cancer cells.
Figure: Photopolymerization of tumor models.
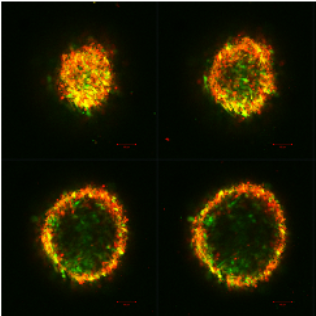
Guiding stem cells to produce cartilage
Despite several decades of research, there are no effective clinical treatment for damaged cartilage in your joints. The underlying mechanisms of attracting stem cells and getting them to turn into the desired cartilage cells are not well understood. I am focusing on investigating the mechanism of stem cell recruitment and chondrogenesis. We are using functionalized polymeric hydrogels and viral reporter constructs in order to track chondrogenic differentiation of mesenchymal stem cells
Figure: Cross sections of pelleted mesechymal stem cells (red) pellet undergoing chondrogenisis (green).