Dr. Derek Hernandez, a recent Schmidt Lab (and Shear Lab) Ph.D. Graduate from UT Austin, recently had his manuscript accepted for publication in the Journal of Materials Chemistry B (JMCB). The paper, “Functionalizing micro-3D-printed protein hydrogels for cell adhesion and patterning”, describes the use of multiphoton excitation to create micro-3D-printed hydrogels and to subsequently modify these structures with functionalized molecules to enhance cell interactions. This paper further demonstrates the ability to create arbitrary immobilized chemical gradient profiles, which could be highly useful for applications, such as nerve repair and other regenerative medicine areas, that benefit from directed cell migration.
Reference:
Hernandez, D.S., E.T. Ritschdorff, S.K. Seidlits, C.E. Schmidt, J.B. Shear (2016). Functionalizing micro-3D-printed protein hydrogels for cell adhesion and patterning. J. Materials Chemistry B.
Abstract:
The extracellular matrix has been shown to profoundly influence both cell morphology and numerous cellular processes – including adhesion, differentiation, and alignment – through a range of chemical, mechanical, and topographical features. In these studies, we investigate a versatile platform for functionalizing micro-3D-printed (μ-3DP) protein hydrogels via multiphoton excitation of benzophenone-biotin, a photoactivatable ligand capable of reacting with the hydrogel matrix, which is subsequently linked to a biotinylated cell-adhesive peptide through a Neutravidin bridge. This functionalization strategy is potentially applicable to a broad range of hydrogel platforms, enabling biomolecules to be precisely patterned at specified locations within 3D materials. As proof-of-concept of this strategy’s utility, we demonstrate that chemical modifications can be made to μ-3DP protein hydrogels that enable Schwann cells to be patterned without altering the mechanical or topographical properties of the hydrogel to an extent that influences SC cell adhesion. The ability to independently control potential cellular cues within in vitro cellular microenvironments is essential to investigating decoupled effects of biomaterial properties on cell-matrix interactions. In addition, we demonstrate feasibility for generating arbitrary immobilized chemical gradient profiles, a result that opens important opportunities for understanding and controlling haptotactic behaviors, such as directed migration, that are key to various tissue regeneration applications.
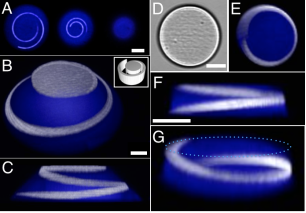 3D patterned protein hydrogels. A) Three confocal slices revealing sub-surface immobilization of NA-TMR within a 10-μm-tall BSA cone. Left image was acquired 1.25 μm above the base of the cone. Each image, left to right, represents a successive axial step of 2.75 μm toward the top of the cone. A Gaussian blur was applied to smooth each image. Scale bar, 10 μm. (B–C) Confocal renderings of a 20-μm-tall BSA cone (blue) labeled with an immobilized spiral of NA-TMR (white) after patterning BP-biotin. 3D rendering of the mask used for BP-b immobilization is inset in panel (B). Scale bar, 10 μm. D) Brightfield image of a 20-μm-tall protein cylinder, and E) a corresponding confocal z-projection. Scale bar, 25 μm. F-G) Confocal renderings of the same BSA cylinder viewed from side (F) and angled (G) perspectives. A dotted light-blue outline in (G) highlights the top of the cylinder. Scale bar, 25 μm
3D patterned protein hydrogels. A) Three confocal slices revealing sub-surface immobilization of NA-TMR within a 10-μm-tall BSA cone. Left image was acquired 1.25 μm above the base of the cone. Each image, left to right, represents a successive axial step of 2.75 μm toward the top of the cone. A Gaussian blur was applied to smooth each image. Scale bar, 10 μm. (B–C) Confocal renderings of a 20-μm-tall BSA cone (blue) labeled with an immobilized spiral of NA-TMR (white) after patterning BP-biotin. 3D rendering of the mask used for BP-b immobilization is inset in panel (B). Scale bar, 10 μm. D) Brightfield image of a 20-μm-tall protein cylinder, and E) a corresponding confocal z-projection. Scale bar, 25 μm. F-G) Confocal renderings of the same BSA cylinder viewed from side (F) and angled (G) perspectives. A dotted light-blue outline in (G) highlights the top of the cylinder. Scale bar, 25 μm