Hudalla Receives R35 Award from the National Institute of General Medical Sciences
October 28, 2019
Gregory Hudalla, associate professor, J. Crayton Pruitt Family term fellow and university term professor, has received a Maximizing Investigators’ Research Award (R35) from the National Institute of General Medical Sciences (NIGMS). NIGMS is a division of the National Institutes of Health (NIH). The $1.8M grant will fund Hudalla’s study of “Glycosylation as a Structural Determinant in Peptide Fibrillization” over the next five years.
Carbohydrates are extraordinarily abundant in the natural world. They make up cellulose in trees and chitin in sea creatures’ exoskeletons. They also decorate all human cells and nearly half of all human proteins, which helps our immune system identify foreign invaders. Yet, “our understanding of the role of carbohydrates in health and disease lags far behind that of all other biomolecules,” said Hudalla, “due to a lack of sophisticated tools and techniques.” Through this award, his research program will close this knowledge gap by developing synthetic analogs of carbohydrate-modified proteins. Specifically, they will create carbohydrate-modified peptides that assemble into fibrillar (strand-like) architectures as a surrogate for highly complex folded proteins. Hudalla and his team will be looking at how carbohydrate appendages influence the folding of peptides into strand-like ‘nanofibers,’ as well as the way these nanofibers function in biological contexts.
Outcomes expected from this award:
- A diverse library of new carbohydrate-modified peptides
- Understanding of how carbohydrates alter the assembly and structure of peptide nanofibers
- New bio-inspired architectures built using peptides and carbohydrates
Hudalla leads the UF Laboratory for Supramolecular Biomaterials and Biotherapeutics, where researchers combine carbohydrates, peptides, and proteins into novel biomedical constructs. Their work provides fundamental insights into how these biomolecules interact with each other in various contexts. In turn, this knowledge enables researchers to predict and manipulate the structure and function of carbohydrates, peptides, and proteins within living systems.



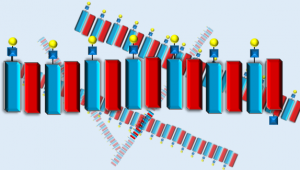


 Cellular and Molecular Bioengineering, an official journal of the Biomedical Engineering Society (BMES), publishes research that advances the study and control of mechanical, chemical, and electrical processes of the cell. Each year, the editors of CMBE dedicate the Young Innovators special issue to highlighting the work of assistant professors conducting innovative bioengineering research at the molecular, cellular, and multi-cellular level. Hudalla’s research focuses on developing new methods to fabricate biomaterials with modular therapeutic or diagnostic capabilities via self-assembly of functional biomolecules, such as proteins and carbohydrates, into precise nano-scale architectures. In the 2016 CMBE Young Investigator issue, Hudalla and co-authors report on their work creating a pair of oppositely-charged synthetic peptides, referred to as “CATCH” (Co-assembly tags based on charge complementarity), to install folded proteins into self-assembling biomaterials. The two CATCH peptides were designed to spontaneously associate into elongated nano-scale fibers when combined in water, yet resist assembly with self-due to like-charge repulsion. This charge-controlled assembly enables efficient microbial production of “fusion proteins” consisting of a CATCH peptide linked to a protein having desirable functional property, such as fluorescence. In turn, CATCH fusion proteins can assemble into functional biomaterials, such as particles and gels, by simply mixing them with the charge-complementary CATCH peptide in water. Hudalla and co-authors envision that this platform will be broadly useful for creating functional biomaterials with interchangeable integrated folded protein components for various biomedical and biotechnological applications.
Cellular and Molecular Bioengineering, an official journal of the Biomedical Engineering Society (BMES), publishes research that advances the study and control of mechanical, chemical, and electrical processes of the cell. Each year, the editors of CMBE dedicate the Young Innovators special issue to highlighting the work of assistant professors conducting innovative bioengineering research at the molecular, cellular, and multi-cellular level. Hudalla’s research focuses on developing new methods to fabricate biomaterials with modular therapeutic or diagnostic capabilities via self-assembly of functional biomolecules, such as proteins and carbohydrates, into precise nano-scale architectures. In the 2016 CMBE Young Investigator issue, Hudalla and co-authors report on their work creating a pair of oppositely-charged synthetic peptides, referred to as “CATCH” (Co-assembly tags based on charge complementarity), to install folded proteins into self-assembling biomaterials. The two CATCH peptides were designed to spontaneously associate into elongated nano-scale fibers when combined in water, yet resist assembly with self-due to like-charge repulsion. This charge-controlled assembly enables efficient microbial production of “fusion proteins” consisting of a CATCH peptide linked to a protein having desirable functional property, such as fluorescence. In turn, CATCH fusion proteins can assemble into functional biomaterials, such as particles and gels, by simply mixing them with the charge-complementary CATCH peptide in water. Hudalla and co-authors envision that this platform will be broadly useful for creating functional biomaterials with interchangeable integrated folded protein components for various biomedical and biotechnological applications.