
Phelps Laboratory
Pancreatic Cellular and Biomaterials Engineering
Overview
The islets of Langerhans are highly complex endocrine mini-organs within the pancreas that secrete insulin and glucagon to control glucose homeostasis and are the target of multiple metabolic diseases including type 1 diabetes (T1D) and type 2 diabetes (T2D). Islet dysfunction and autoimmunity cause severe morbidity in more than 300 million people worldwide and are associated with health care costs in the hundreds of billions of dollars annually.
In T1D, the insulin-producing beta cells of the islet are destroyed by an auto-immune attack. Our research interests lie at the interface of biomaterials engineering and the biology and treatment of diseases of the pancreatic islets such as T1D.
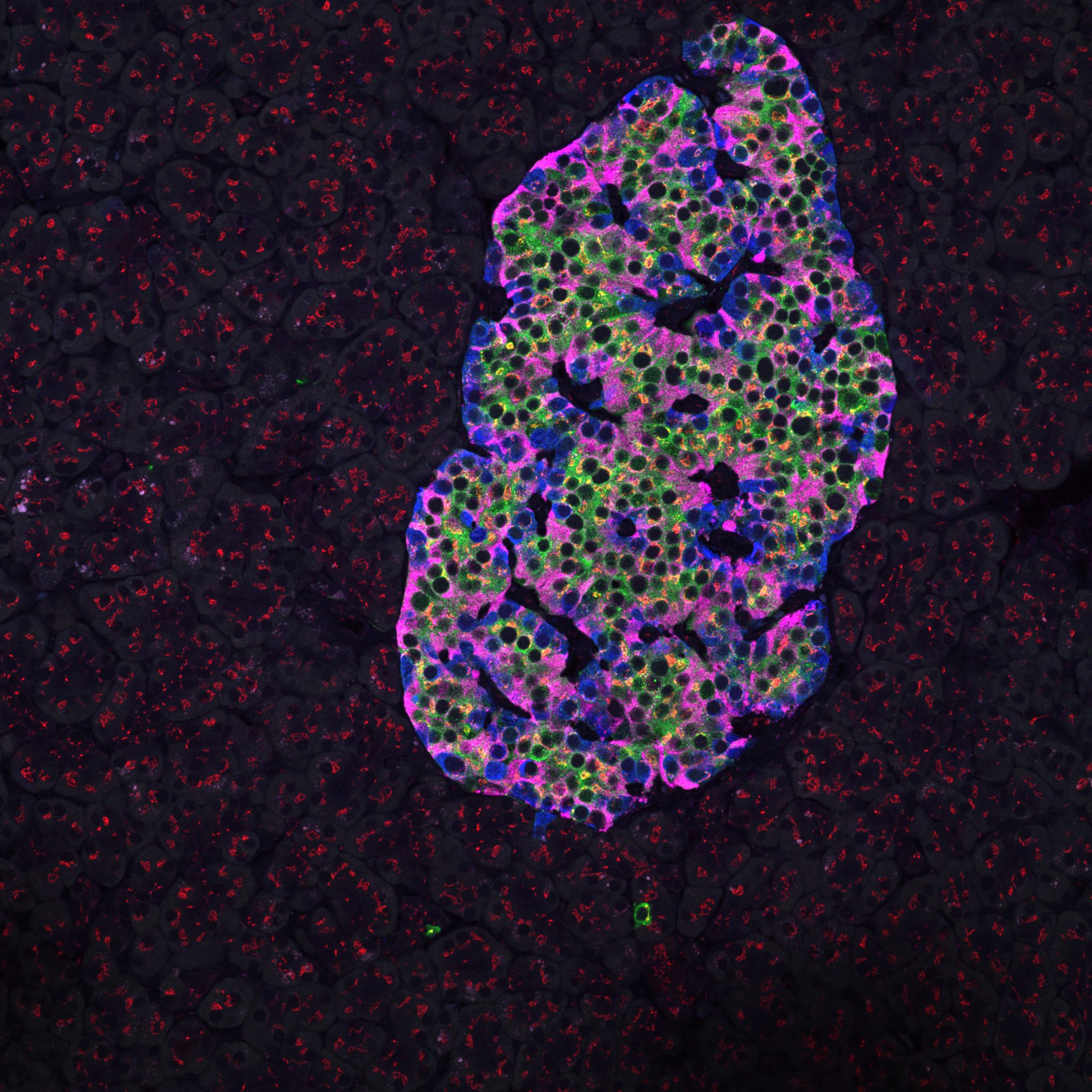
Healthy human pancreatic islet
Immunostaining for insulin, glucagon, GAD65, and giantin
Biomaterials for T1D Immune Engineering
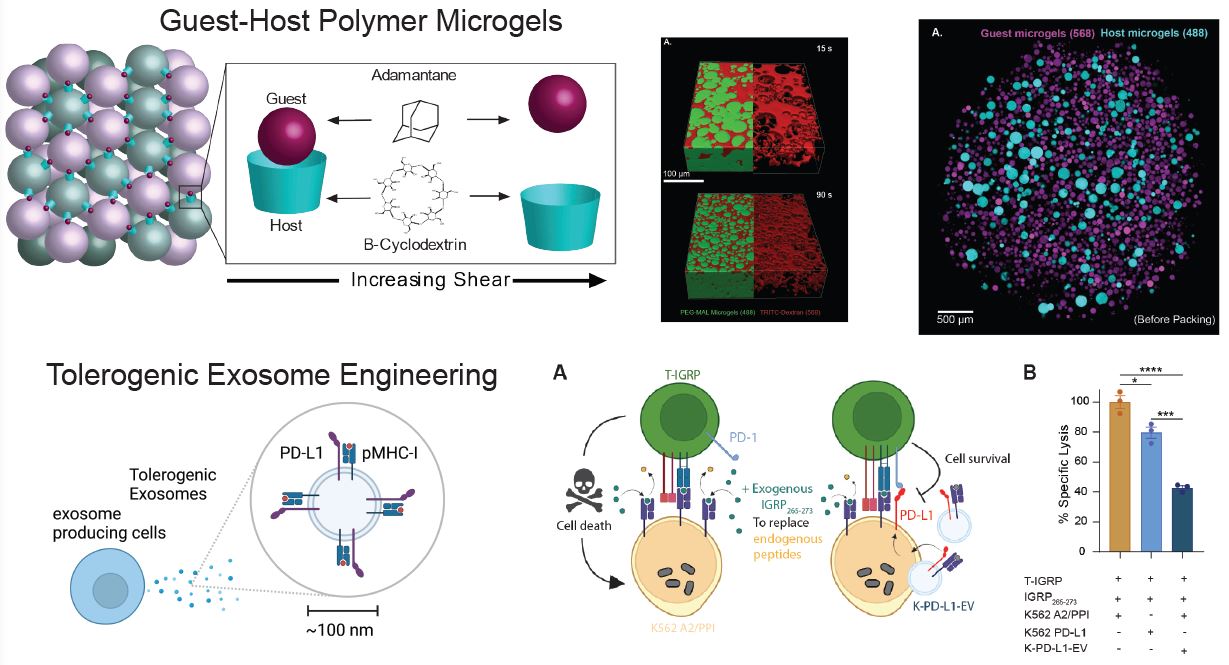
We previously developed polyethylene glycol (PEG) hydrogel matrices for revascularization of transplanted pancreatic islets. Now, we are progressing this technology to generate microporous annealed particle (MAP) hydrogels composed of packed hydrogel microspheres to create interstitial porosity in the gel structures. The microspheres are functionalized with guest-host molecules so that they interact reversibly with each other and the material becomes shear-thinning and self healing.
We are engineering approaches to modulate the immune system towards beta cell tolerance by functionalizing the surfaces of MAP hydrogels with proteins that inhibit T cell activation. In another application, we are designing extracellular vesicles (also known as exosomes) to present negative immune modulators together with beta cell antigens.
Living pancreas tissue slices
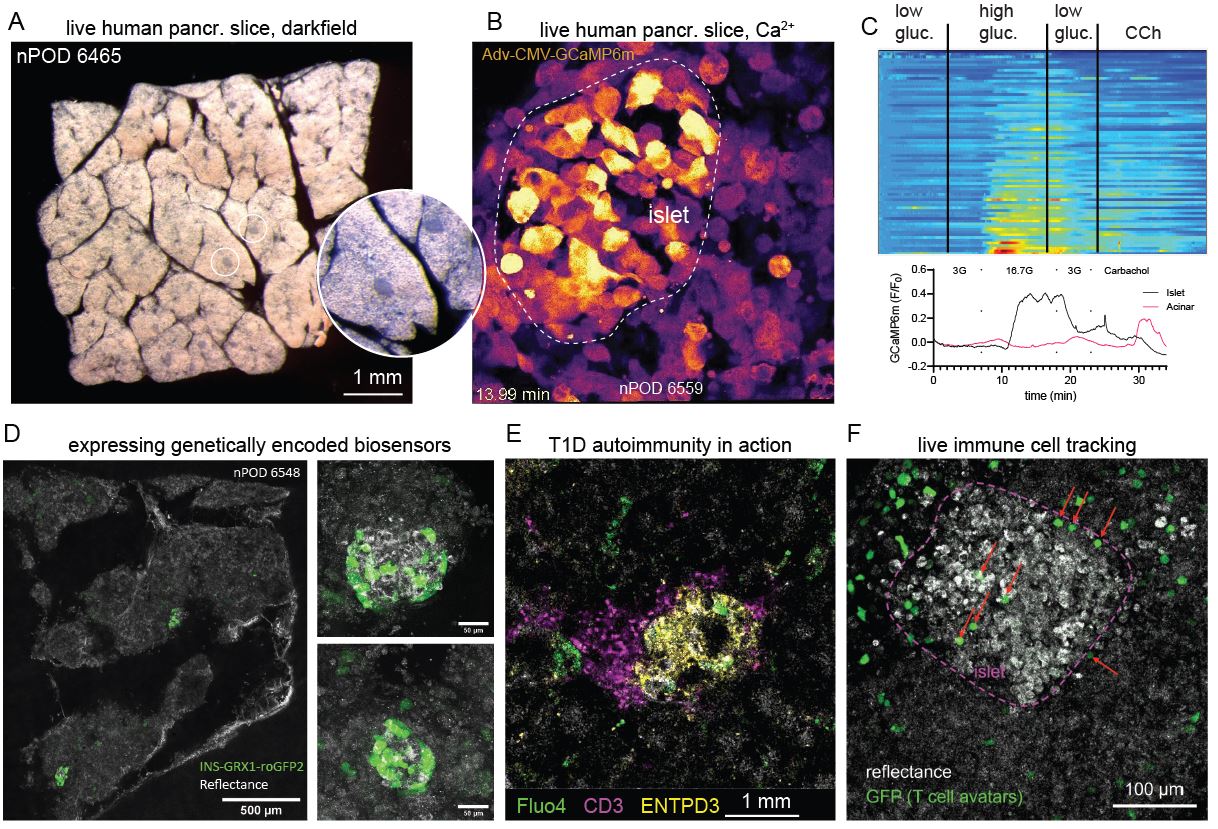
Type 1 diabetes (T1D) is an autoimmune disease characterized by circulating autoantibodies and T cell mediated destruction of insulin-producing beta cells. We seek to observe the process of T1D in the native environment of the pancreas. Such an approach allows us to probe the function of beta cells in situ while they are undergoing autoimmune attack.
Islet Autocrine and Paracrine Signaling
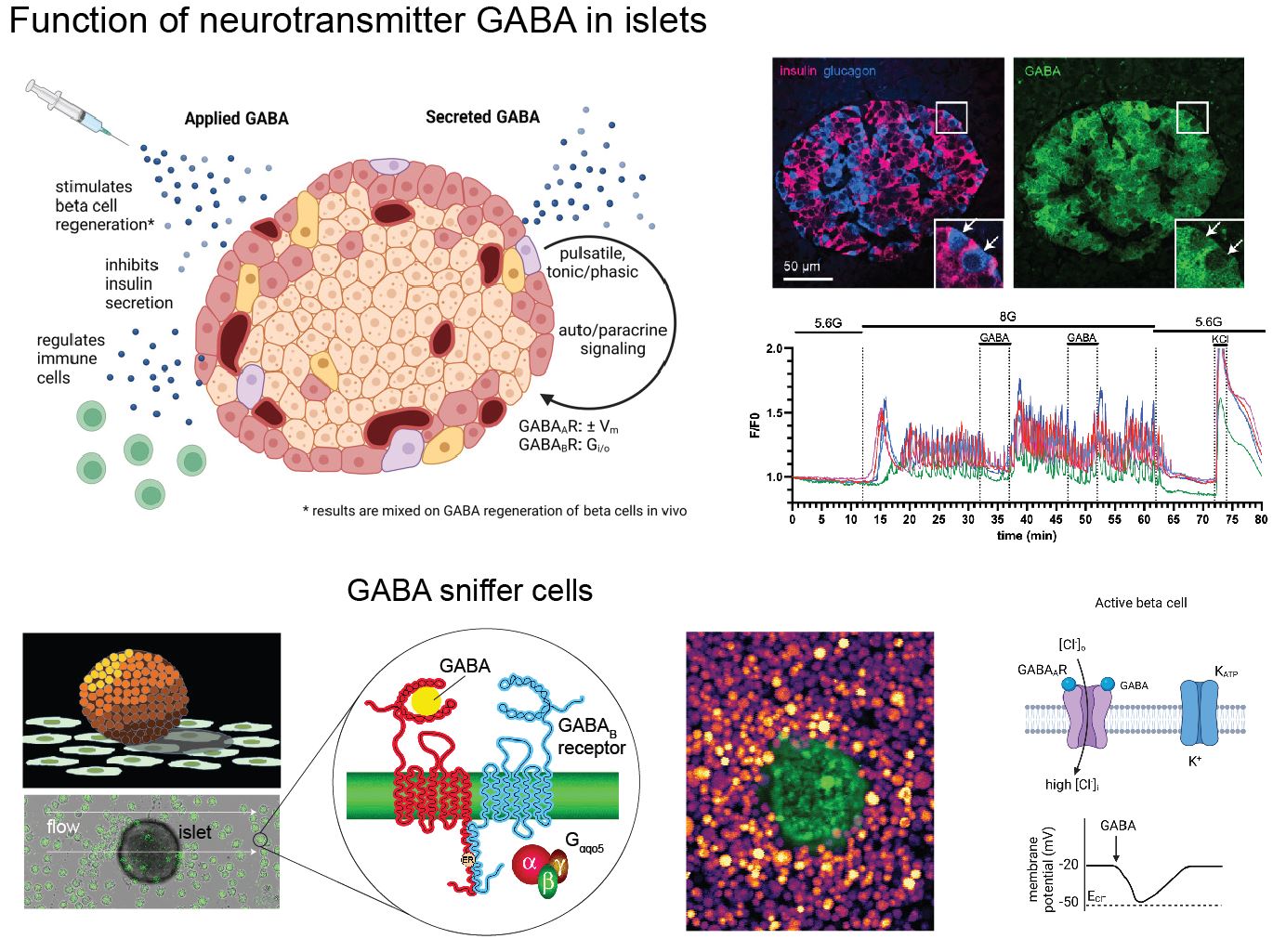
One of the targeted beta cell membrane proteins in human T1D is GAD65, the smaller isoform of the GABA synthesizing enzyme glutamic acid decarboxylase (GAD). Interestingly, GAD65 synthesizes the inhibitory neurotransmitter GABA. GABA biosynthesis is fairly unique to beta cells and neurons. We are studying the islet GABA system to comprehend how GABA contributes to islet function in normal and disease states.
© 2023 by Edward A. Phelps


